From U.S. Department of Defense, Washington
Statement attributed to Lt. Col. Mike Andrews, Department of Defense spokesman: "Today the Department of Defense and the U.S. Department of Health and Human Services, announce a $138 million contract with ApiJect Systems America for “Project Jumpstart” and “RAPID USA,” which together will dramatically expand U.S. production capability for domestically manufactured, medical-grade injection devices starting by October 2020.
Spearheaded by the DOD’s Joint Acquisition Task Force (JATF), in coordination with the HHS Office of the Assistant Secretary for Preparedness and Response, the contract will support “Jumpstart” to create a U.S.-based, high-speed supply chain for prefilled syringes beginning later this year by using well-established Blow-Fill-Seal (BFS) aseptic plastics manufacturing technology, suitable for combatting COVID-19 when a safe and proven vaccine becomes available.
Editor's notes:
President Trump will mobilize military to distribute vaccine, when available.
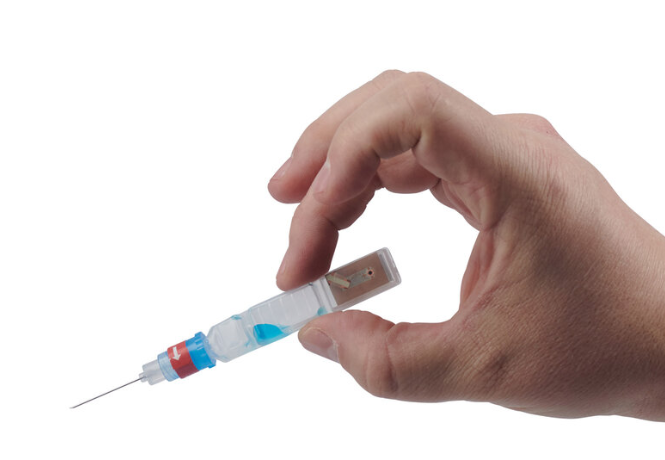
ApiJect is a founding member of the Rapid Consortium. A YouTube video produced by the organization states, "These facilities will make enough prefilled syringes to inject every man, woman, and child in America with just the right dose 30 days after a vaccine becomes available. Plus every prefilled syringe can have an RFID chip attached. This will allow healthcare workers to use their mobile phones to automatically capture where and when every injection takes place, helping public health officials make more informed decisions."
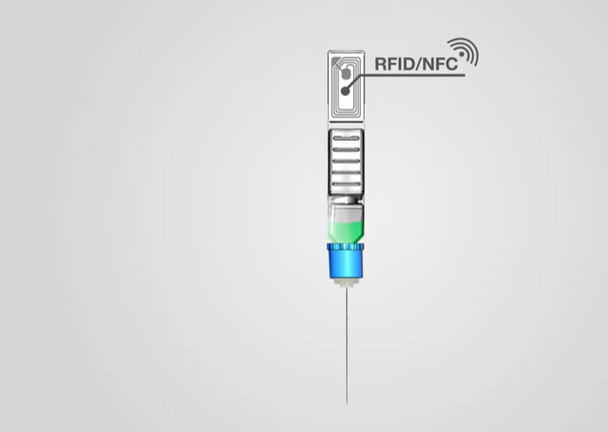
On it's website, ApiJect explains:
A Digital “Snapshot” for Every Injection
Whether health officials are running a scheduled vaccination program or an urgent pandemic response campaign, they can make better decisions if they know when and where each injection occurs. With an optional RFID/NFC tag on each BFS prefilled syringe, ApiJect will make this possible. Before giving an injection, the healthcare worker will be able to launch a free mobile app and “tap" the prefilled syringe on their phone, capturing the NFC tag’s unique serial number, GPS location and date/time. The app then uploads the data to a government-selected cloud database. Aggregated injection data provides health administrators an evolving real-time “injection map.”
[end of editor's note]
The Department of Defense statement continues:
By immediately upgrading a sufficient number of existing domestic BFS facilities with installations of filling-line and technical improvements, “Jumpstart” will enable the manufacture of more than 100 million prefilled syringes for distribution across the United States by year-end 2020.
The contract also enables ApiJect Systems America to accelerate the launch of RAPID USA manufactured in new and permanent U.S.-based BFS facilities with the ultimate production goal of over 500 million prefilled syringes (doses) in 2021. This effort will be executed initially in Connecticut, South Carolina and Illinois, with potential expansion to other U.S.-based locations. RAPID will provide increased lifesaving capability against future national health emergencies that require population-scale vaccine administration on an urgent basis.
RAPID’s permanent fill-finish production capability will help significantly decrease the United States’ dependence on offshore supply chains and its reliance on older technologies with much longer production lead times. These supplies can be used if a successful SARS-COV-2 vaccine is oral or intranasal rather than injectable." [end DOD release]

News Release From ApiJect Systems:
ApiJect Systems America, Inc., a public benefit corporation based here, today announced that it has been awarded an HHS-DOD Title 3, DPA contract valued up to $138 million to accelerate the building of a new U.S.-based, high-speed, population-scale emergency drug injection capability with prefilled syringes from its subsidiary RAPID USA Inc. RAPID USA's emergency program, "Project Jumpstart" is being initiated to supply 100 million prefilled syringes by year-end.
RAPID USA's Project Jumpstart will immediately contract with a sufficient number of existing U.S.- based Blow-Fill-Seal (BFS) facilities to install filling lines and technical upgrades to enable production of prefilled syringes before year-end. BFS is a well-established high-speed medical- grade plastics aseptic manufacturing process that specializes in the high-volume production of pharmaceutical products. Jumpstart will also purchase and stockpile 100 million Needle Hubs for ApiJect prefilled syringes. Jumpstart will develop the capability to manufacture a minimum of 30 million prefilled syringes per month once therapeutic drugs and vaccines become available.
In parallel with Project Jumpstart, RAPID USA will build a network of 30 U.S.-based BFS manufacturing lines at three different, geographically dispersed, sites. Once operational, these 30 lines will fill, finish, and package up to 330 million prefilled BFS syringes per month. Initial production will begin in late 2021. RAPID USA will also build a U.S.-based training and prototyping facility capable of supporting 500 U.S.-based jobs at RAPID USA's three manufacturing sites.
ApiJect Systems America CEO Jay Walker commented: "ApiJect's Title 3, DPA funding gives our subsidiary RAPID USA the capability to swiftly create the domestic surge capacity in prefilled syringes that will be needed as therapeutics and vaccines become available. Project Jumpstart is the first stage in RAPID's HHS-DOD supported two-stage effort. Within six months, Project Jumpstart will create a surge capacity to supply 100 million prefilled syringes and more than 500 million in 2021. Stage two, running in parallel with Jumpstart, will have RAPID USA building a network of 30 U.S.-based BFS manufacturing lines, enabling a monthly production of up to 330 million BFS prefilled syringes."
Walker continued: "When discussions with HHS ASPR first began last year ApiJect was then focused on global health, specifically injection safety in low and middle-income countries where needle reuse and contaminated multi-dose vials kill as many as two million people every year and infect 10 million or more with transmissible diseases such as HIV and Hep-C. ASPR's leadership wanted us to turn our attention to building a U.S.-based population-scale surge capacity for flexible biodefense purposes. We started immediately, and when COVID-19 emerged as a pandemic threat, our public-private partnership with HHS, which had been created in January, accelerated to focus on building both an emergency capability as well as longer-term sustainable injection surge capacity."
Walker further commented: "RAPID USA is led by our multi-disciplinary team of experienced engineers, pharmaceutical technology experts, and management leadership. Our team is expending extraordinary efforts to ensure that when drugs are developed and tested all Americans can receive critical injections. We will have done our part by providing the manufacturing capacity to support the necessary volume of ready-to-use prefilled syringes that contain essential medicines, be they therapeutics or vaccines. Our public-private partnership, supported by Jefferies Financial Group, and the HHS-DOD Title 3 contract, demonstrates the vital role that RAPID will play in the war against COVID-19, as well as future national health emergencies."
Rich Handler, CEO and Brian Friedman, President of Jefferies Financial Group, Inc., commented: "Finding a solution to the COVID-19 crisis demands the best from each of us, as companies and as individual citizens. When we learned what ApiJect was doing with the U.S. Government, Health and Human Services and the Department of Defense, we saw a role where Jefferies and our nearly 4,000 global professionals could make a difference. We invested in RAPID USA as we believe it is the right step at the right time, and we will continue to support ApiJect to assure RAPID USA can do their important job of building the surge capacity needed here on U.S. soil to help put this crisis behind us."
ABOUT APIJECT AND RAPID USA
ApiJect Systems America, Inc., is a public benefit corporation dedicated to making injectable medicines safe and available for everyone. By building a network using high-speed, high-volume Blow-Fill-Seal medical grade plastics technology and an interlocking Needle Hub, ApiJect can supply hundreds of millions of ultra-low-cost prefilled syringes with optional RFID tags to enable GPS-based mobile tracking. ApiJect, along with the U.S. Department of Health and Human Services, is a founding member of the RAPID Consortium, a public-private partnership dedicated to giving the U.S. and the world the surge drug packaging it needs for addressing future pandemics and bio-emergencies. Learn more about ApiJect at www.apiject.com.
RAPID USA, Inc., a subsidiary of ApiJect Systems America, Inc., is building and will manage the high-speed, high volume surge capacity for drug fill, finish and packaging that America needs to effectively respond to future pandemics and bio-emergencies. Starting in the second half of 2021, RAPID USA will begin rolling out new U.S.-based BFS drug packaging lines that once completed in 2022, will provide the capacity to fill and finish up to 330 million prefilled syringes per month for the U.S. and the world. The HHS-DOD emergency program, Project Jumpstart, to supply the U.S. with 100 million BFS prefilled syringes by year-end, is a RAPID USA initiative. Learn more at www.rapidconsortium.com.